Natural Fibers Are Continuing to Make Inroads Into a Sector Dominated by Man-Made Fibers
With increasing concerns over their environmental impact, medical textiles from natural renewable resources such as natural fibers, particularly where these products remain in close contact with the human body, are continuing to find favor owing to their biological compatibility, biological degradability, perme
ability and nontoxicity.
Both specialty and commodity fibers are used in the manufacture of medical and hygiene textiles. Specialty fibers include such materials as chitosan, chitin, collagen and calcium alginate fibers, while commodity fibers can be further subdivided into natural fibers, such as cotton, silk and viscose, and synthetic fibers, such as polyester, polyamide, polypropylene and polytetrafluoroethylene.
These fibers can be biodegradable or non-biodegradable, depending on the application, with the resulting medical textile products being either reusable or disposable.
Sustainable Uses for Cellulose
Cellulose is one of the most abundant biodegradable materials in nature and has been widely used in medical applications such as wound dressings, tissue engineering, controllable drug delivery and blood purification owing to its biocompatibility, hydrophilicity, biodegradable, non-toxicity and antimicrobial properties.
Lithuanian scientists have now developed a production method for a nanofibrous cellulose matrix, which has the potential for use in non-renewable biomedical applications.
The cellulose nanofiber was developed by researchers at the Faculty of Chemical Technology, Kaunas University of Technology, using the wet-type electrospinning method, in which cellulose is dissolved in ionic liquids and the solution converted into fibers.
As well as the use of “green solvents,” the raw material for this process can be either raw cellulose or cellulose waste. Depending on the purity of the material, the resulting fiber can be used for different biomedical products.

For example, recycled cellulose can be used to produce new polymer composite products, including biomedical applications where this type of nanofibrous structure has unique biocompatibility properties.
So far, the use of cellulose in tissue engineering has been tested for the reconstruction of cartilage, bone and vascular structures, according to Ingrida Pauliukaityte, a Ph.D. student at the university.
Biodegradable Superabsorbent Made From Hemp
Researchers at the Purdue Research Foundation, West Lafayette, Indiana, USA, are using cellulose extracted from hemp and refined through treatments to create superabsorbent materials, which could find applications in various industries, including disposable diapers and feminine hygiene products.
The research team, led by Professor Senay Simsek, head of the university’s Department of Food Science, utilized cellulose extracted from the highly absorbent hurds (the inner woody core) and the less absorbent but strong and durable bast (the fibrous outer layer) of the hemp plant, which were then refined through a patented sequence of treatments. This process enhances the surface area and porosity of the cellulose, significantly increasing its water absorption and retention capabilities.
The researchers tested the new superabsorbent materials made using standardized absorbency tests, comparing them with traditional materials. The hemp hurds showed higher absorption capacity than both hemp bast and many traditional materials.
Artificial Spider Silk
Chinese scientists have developed artificial spider silk that consists of proteins and can heal chronic wounds. The artificial silk was strong enough to be woven into bandages that helped treat joint injuries and skin lesions in mice, according to an article published by the American Chemical Society.
The researchers, based at the School of Pharmaceutical Sciences, Nanjing Tech University, aimed to modify the natural protein sequence to design an easily spinnable, yet still stable, spider silk using microbes.
The team first used these microbes to produce the silk proteins, adding extra peptides, which helped the artificial silk proteins form an orderly structure when folded and prevented them from sticking together in solution, increasing their yield.
Then, using an array of tiny, hollow needles attached to the nozzle of a 3D printer, the researchers drew the protein solution into thin strands in the air and spun them together into a thicker fiber.
They then wove their artificial silk fibers into prototype wound dressings that they applied on mice with osteoarthritis and chronic wounds caused by diabetes. Drug treatments were easily added to the dressings, and the team found that these modified dressings boosted wound healing better than traditional bandages.
PLA Fibers with Antibacterial Properties
Spanish scientists have developed new biocompatible fibers with antibacterial properties. Made from polylactic acid (PLA), a biodegradable and degradable polymer, and reinforced with magnesium nanoparticles, these fibers show potential for use in medical implants, improved healing and reduced infection risk in a wide range of medical applications.
To create the fibers, the researchers from the Instituto de Ciencia y Tecnología de Polímeros and Centro Nacional de Investigaciones Metalúrgicas used electrospinning, with PLA as the base material, to which they incorporated magnesium oxide and magnesium hydroxide nano-particles in gradually increasing concentrations, ranging from 0.5% to 20%.
The researchers observed promising signs for the new PLA-based electrospun fibers for bone repair and for combating infections. Further, the biocompatibility and potential versatility of these fibers suggest a wider range of applications, such as wound dressings, tissue engineering scaffolds or advanced sutures.
Dressings That Prevent Infection
Wound dressings are important for wound care and management, but there is little evidence to support the routine use of current antimicrobial dressings for complex wounds, which aim to prevent or stop the growth of microbes.
Most antimicrobial dressings do not show clear advantages, which could be due to the active agents preventing wound healing or not working against certain microbes. Further, the repeated or prolonged use of traditional antibiotics in wound care, especially chronic wound treatments, can cause the emergence of bacteria, fungi and viruses resistant to these commonly used antimicrobials.
To address these issues, a multidisciplinary team in the UK is working to develop a new type of wound dressing that could prevent wound infection and help improve healing.
Headed by Professor Jason Wong of the NIHR Manchester Biomedical Research Centre, the researchers are aiming to develop new dressings that contain antimicrobial peptide (AMP) releasing hydro-
fibers. Unlike traditional antibiotics, AMPs are effective against a wide range of microbes, can act quickly and penetrate through microbes’ resistant barriers.
The team has already developed an AMP that has been tested against a range of microbes, is easy to produce and is non-toxic. The study will aim to show that it has low side effects and develop it towards a clinical product.
For the fiber sheath the team chose polycaprolactone (PCL), a biocompatible and biodegradable polymer that is already being used successfully in the medical field.
Covered Shape-Memory Polymeric Fibers
As well as natural fibers, developments in polymeric fibers continue to be announced. For example, shape-memory polymeric fibers (SMPFs) for medical textile applications, such as body support bandages and orthotics, where shape fixity and recovery can be kept constant over multiple shape-memory cycles, have been developed by the Helmholtz-Zentrum Hereon, a research institute in Geesthacht, Germany.
The covered SMPFs have a core fiber of semi-crystalline polymer and an unstretchable covering yarn wound around it such that the maximum engineering strain of the core shape-memory fiber is reduced to at most the strain at the yield point of the uncovered core fiber. As a result, the covering yarn is used to limit the stretchability or deformation of the core shape-memory fiber during programming to ensure maximum recoverable strain.
Essentially, this structure prevents overstretching of the core fiber, causing its irreversible plastic deformation and deterioration of mechanical and shape-memory properties as well as shape. The semi-crystalline core fiber can be selected from a range of shape-memory polymers, such as a polyethylene-co-vinyl acetate.
The SMPFs developed by Hereon will be particularly useful for medical applications, such as compression garments, orthopedic bandages, orthoses, posture correction garments, push-up garments, corsets and corsages, and sports garments.
“Liquid Core” Polymer Fibers
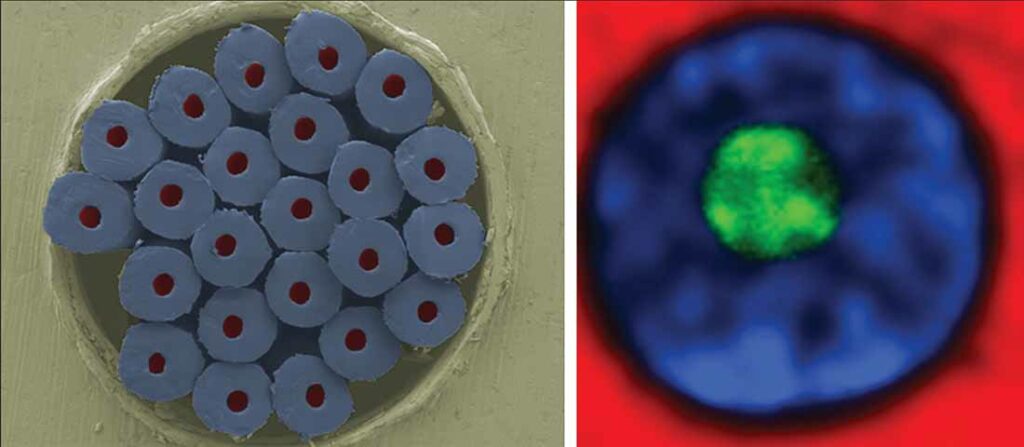
Researchers at Empa, the Swiss Federal Laboratories for Materials Science and Technology, are developing polymer fibers that can deliver active ingredients precisely over the long term. These “liquid core fibers” contain drugs inside and can be processed into medical textiles.
The aim is to produce medical textile products with special capabilities, such as surgical suture materials, wound dressings and textile implants that can administer painkillers, antibiotics or insulin precisely over a longer period of time. Another aim is to achieve individual, patient-specific dosage of the drug in the pursuit of personalized medicine.
For the fiber sheath the team chose polycaprolactone (PCL), a biocompatible and biodegradable polymer that is already being used successfully in the medical field. The fiber sheath encloses the active substance, such as a painkiller or an antibacterial drug, and releases it over time.
Led by Dr. Edith Perret from Empa’s Advanced Fibers laboratory in St. Gallen, the researchers have produced PCL fibers with a continuous liquid core by means of melt spinning. In initial laboratory tests, stable and flexible liquid-core fibers were produced.
Together with a Swiss industrial partner, the Empa team demonstrated that this process not only works in the laboratory but also on an industrial scale. The parameters according to which the medical fibers release an enclosed agent were first investigated using fluorescent model substances and then with various drugs.
With diameters of 50–200 µm, the medical fibers are large enough to be woven or knitted into robust textiles. However, they could also be guided inside the body to deliver hormones such as insulin, said Perret. Another advantage is that fibers that have released their medication can be refilled.
In the next step, the researchers want to equip surgical suture material with antimicrobial properties. The new process will be used to fill various liquid core materials with antibiotics in order to suture tissue during an operation in such a way that wound germs have no chance of causing an infection.
What is Not Working
y Vaginal Mesh Implants
The “vaginal mesh scandal” – a global health crisis involving the use of mesh implants to treat urinary incontinence and pelvic organ prolapse, often experienced by women after childbirth – continues to rumble on.
Made from polypropylene (PP), the inert mesh is permanent and not designed to be removed; removal surgery can damage nerves and organs. However, complications include vaginal bleeding, discharge, pain and difficulty having intercourse, with some women left in constant pain, unable to walk, work or have sex. Further, many women say they were not told about the potential risks of the implants by their surgeons.
In the U.S., thousands of women have sued the mesh manufacturers and received billions of dollars in payouts. In the UK, more than 800 women are suing the country’s National Health Service and manufacturers, including Johnson & Johnson.
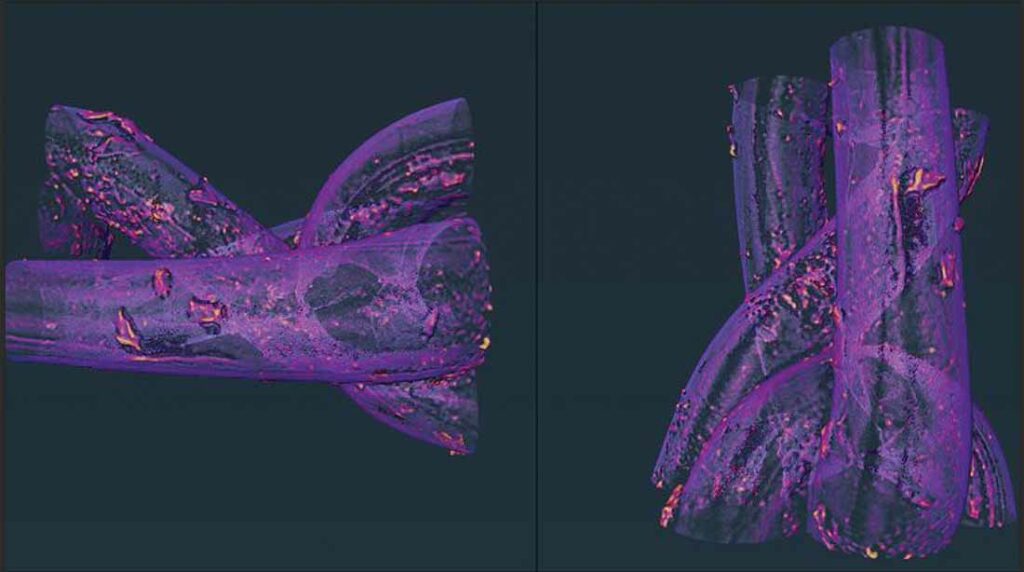
New evidence of the failings of the PP mesh material has now been revealed in a study by researchers at the UK’s University of Sheffield, which showed that the material can begin to degrade within 60 days of being implanted. This degradation increased further in materials implanted for up to 180 days.
Of particular concern to the researchers was the discovery of PP particles within the tissue surrounding the implantation site. The concentration of these particles was significantly higher – more than 10 times greater – after 180 days than at 60 days.
y Toxic Metals Detected in Tampons
The U.S. Food and Drug Administration has launched an independent review into any possible effects of toxic metals found in tampons. This follows the release of a study last July by researchers at the University of California Berkeley, who found traces of arsenic, lead, cadmium and other metals in 30 tampons from 14 brands obtained from major online retailers and stores in the U.S., the UK and Greece.
The research team highlighted significant health risks associated with their use: metal absorption has been found to heighten the risk of dementia, infertility, diabetes and cancer. Such toxic metals can damage the liver, kidneys and brain, as well as the cardiovascular, nervous and endocrine systems. In addition, these metals can harm maternal health and fetal development.
Lead and arsenic were notably present among the toxic metals detected, with organic tampons showing higher arsenic levels and non-organic ones containing more lead. The study also found that products sold in the U.S. had higher concentrations of lead than those marketed in the Europe.


