By Donald Wagner and Joel Zazyczny, Gelest, Inc.
BIOSAFE® is a new silicon-based antimicrobial from Gelest, Inc. Its novel activity imparts long lasting bacteriostatic, fungistatic and algistatic properties to substrates, such as textiles, preventing deterioration and discoloration caused by fungi. Furthermore, BIOSAFE antimicrobials prevent algae growth and inhibit the growth of odor-causing bacteria. This new generation of antimicrobial can be incorporated into and applied onto any textile, and is therefore ideal for fabrics that are susceptible to microbial contamination.
BIOSAFE antimicrobials come both in powder form for use as a functional additive in spun fibers, and as a water-based, VOC-free solution for use in coating textiles. BIOSAFE’s reactive silicon-based antimicrobial chemistry can be deposited into or onto the fiber for durable, leach-resistant, and non-migrating microbiostatic properties. The antimicrobial works in such a way that it does not promote the development of resistant microorganisms.
With proper integration, BIOSAFE products exhibit high antimicrobial performance, which reduces odors and discoloration from bacteria, fungi (mold and mildew) and even algae, keeping textiles safer and cleaner. The patented polymeric chemistry of the antimicrobial has a long track record of success and is a significant advancement over chemistry currently in the
marketplace.
Microbes can damage natural and synthetic fibers. They can stain, produce odors, and compromise the strength and integrity of the textile. The silicon-based antimicrobial is added during processing to protect the finished product.
Fibers and the textiles into which they are woven are susceptible to attack from microbes, depending on the type of substrate, as well as the presence of moisture, temperature and exposure to other environments.
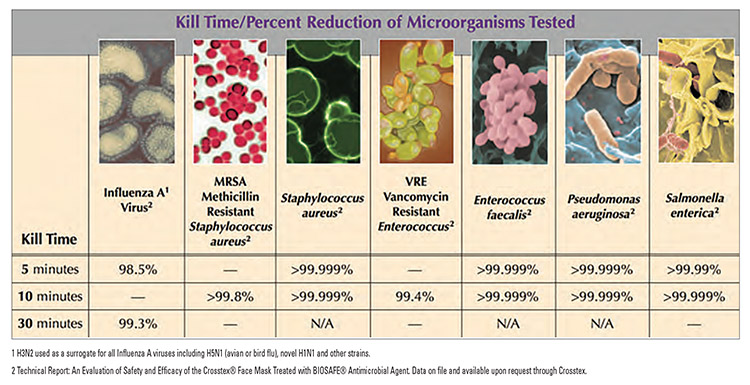
It is always a good practice to test antimicrobial action against common bacteria such as E.coli, P.aeruginosa, and S.aureus. Gelest has tested the efficacy of treated textiles using ASTM-E2149-10, which confirms excellent long-lasting antimicrobial activity. Other test methods utilized for testing the efficacy of antimicrobial activity on textiles are (AATCC) Methods 147, 30 and 100. However, these methods provide no dynamic action to test the potential of a non-leaching active such as BIOSAFE. Hence, ASTM E2149-10 is preferred.
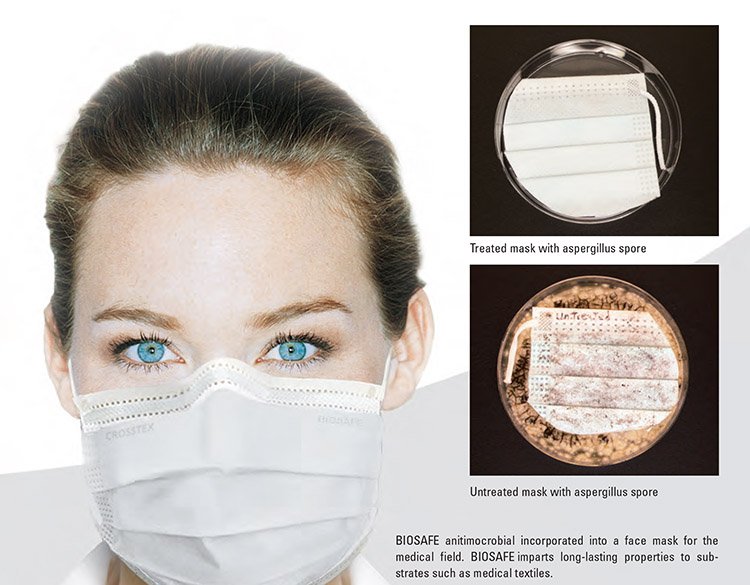
The BIOSAFE technology can be added to control pathogenic organisms that may be present on the surface of the fibers. Crosstex, a Cantel Medical Company, sells a BIOSAFE-treated medical face mask made of polyolefin nonwoven material for such use. Crosstex sells the facemask outside of the U.S. only. The chart below shows the results of testing done on the Crosstex-BIOSAFE mask.
Antimicrobials that compete with BIOSAFE technology include silver zeolites and nano-sliver, triclosan, zinc pyrithione, and copper or zinc napthanate. All of these active ingredients can kill microbes in the appropriate dosage, but fall short of being safe and non-toxic.
Acute toxicity testing is done under GLP and measures adverse effects within a short time after administration of a dose of the test substance, or of multiple dosages given within 24 hours. Signs of toxicity are revealed through acute toxicity studies. Dose levels leading to death or serious untoward effects are then measured, classified and labeled. Clearly, BIOSAFE antimicrobials displayed no significant toxicological effects in these studies.
The toxicity tests reported were performed on the pure BIOSAFE active powder under GLP protocol at the following independent laboratories: Stillmeadow Laboratories, NAMSA and Toxikon. As part of the U.S. Environmental Protection Agency (EPA) registration of the BIOSAFE antimicrobial agent, the following tests have been completed:
Acute Dermal Toxicity in Rabbits OPPTS No. 870.1200
“The test substance HM 4100 was evaluated for its acute dermal toxicity potential and relative skin irritancy when administered to albino rabbits. The acute dermal LD50 as indicated by the data is greater than 5050 mg/kg in males and females.”
Acute Dermal Irritation in Rabbits OPPTS No. 870.2500
“The primary irritation index of 0.3 out of a possible 8.0 was obtained from the 1, 24, 48, and 72 hour observations and was used to give HM 4100 a descriptive rating of slightly irritating. Based on the 72 hour observation only, HM 4100 is assigned to Toxicity Category IV.”
Acute Eye Irritation in Rabbits OPPTS 870.240
“Based on the maximum average eye irritation score of 5.3, the test substance HM 4100 is rated mildly irritating. Since all positive effects cleared prior to 48 hours, the test substance is rated mildly irritating and assigned a Toxicity Category III. No irritation was observed in all eyes on day 4.”
Acute Oral Toxicity Study (UDP) in Rats OPPTS 870.1100
“The test substance, HM 4100, was evaluated for its acute oral toxicity potential when
administered to albino rats. The acute oral LD50 is estimated to be greater than 5000 mg/kg in females.”
Acute Inhalation Toxicity Study in Rabbits OPPTS 870.130
“HM 4100 was evaluated for its acute inhalation toxicity potential in albino rats. As indicated by the data, the acute inhalation LC50 is greater than 2.19 mg/L in males and females.”
Skin Sensitization: Local Lymph Node Assay in Mice OPPTS 870.2600
“HM 4100 produced a stimulation index of < 3 in all groups of test animals, and is not therefore considered a sensitizer.”
The BIOSAFE HM4100 antimicrobial agent was also subjected to biocompatibility tests. These results confirm that it meets ISO 10993 and/or USP requirements. The following results were supplied by NAMSA to establish the biocompatibility of BIOSAFE.
ISO Intracutaneous Study – Extract, ISO 10993: Biological Evaluation of Medical Devices, Part 10: Test for Irritation and Delayed-Type Hypersensitivity. The material extracts met the requirements of the test. There was no significant difference between the mean score of the test extracts and the mean score of the corresponding controls.
USP and ISO Systemic Toxicity Study – Extract, United States Pharmacopeia and ISO 10993: Biological Evaluation of Medical Devices, Part 11: Tests for Systemic Toxicity (ISO). Each test article extract met the test requirements. Under the conditions of this study, there was no mortality or evidence of systemic toxicity from the extracts.
Cytotoxicity Study Using the ISO Elution Method (IX MEM Extract), ISO 10993: Biological Evaluation of Medical Devices, Part 5: Test for Cytotoxicity, in vitro Methods guidelines. Under the conditions of this study, the IX MEM test extract showed no evidence of causing cell lysis or toxicity. The IX MEM test extract met the requirements of the test since the grade was less than 2 (mild reactivity).
Not only has BIOSAFE technology demonstrated significant potency in its antimicrobial activity, but also very significantly this is coupled with low toxicity and biocompatibility – a rare combination that delivers safety and efficacy.
Looking forward to the process of product development, several key factors need to be considered when commercializing and launching a new antimicrobial textile:
• Compatibility: As a fiber or textile surface treatment the BIOSAFE active provides antimicrobial activity. As an additive for fiber spinning, for example, the compound provides strong antimicrobial activity when spun into polyolefins (i.e., PE) and nylons (i.e., PA6), but less activity in polyester.
• Antagonists: Anionic surfactants, excessive temperature, and compatibility with other chemicals such as flame-retardants have to be considered.
• Marketing claims: Understanding the value the end textile or garment is to deliver is important in determining the appropriate testing and regulatory approvals. For example, one textile might be designed to eliminate odors and another might be designed into an air filter to control microbes.
• Durability: While surface-treating a textile with a BIOSAFE product provides good antimicrobial activity, spinning HM4100 into the fiber itself provides durability and permanent protection from laundering because the active is dispersed throughout the polymer matrix. Each method has its own advantages and disadvantages.
• Spectrum of activity: What microbe will the textile face? Testing relevant microorganisms (bacteria, fungi, algae, viruses or yeast) for a particular application makes for a smooth transition to commercialization. A list of microbes against which the BIOSAFE technology has been tested is available to customers upon request.
• Speed of action: Some applications simply inhibit microbes whereas others, such as the Crosstex facemask, require fast antimicrobial activity.
• Cost effectiveness: BIOSAFE antimicrobial is used in small percentages on the finished good (less than 1% by weight, and in some instances less than 0.1%) and delivers antimicrobial activity at a competitive cost.
References
D’Antonio NN, Rihs JD, Stout JE, Yu VL. Computer keyboard covers impregnated with a novel antimicrobial polymer significantly reduce microbial contamination. American Journal of Injection Control 2013; 41:337-9.
Medical Face Masks Treated with BIOSAFE. Available from: http://www.crosstex.com/pdfs/BIOSAFE_Brochure.pdf. Accessed August 31, 2015.
Patent Nos. 6,572,926; 6,146,688; 7,851,653; 7,858,141.
For more information contact:
Gelest, Inc.
11 E. Steel Road
Morrisville, Pennsylvania 19067
Tel: 215-547-1015
Fax: 215-547-2484
Email: dwagner@biosafe.com
Website: www.gelest.com

